
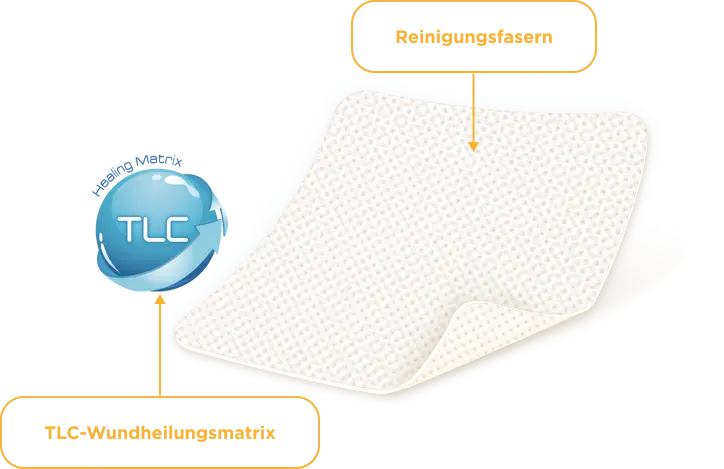
Mit den innovativen Wundauflagen UrgoClean Kompresse und UrgoClean Tamponade sind Sie auf der sauberen Seite. Die UrgoClean Kompresse besteht aus polyabsorbierenden Reinigungsfasern und ist mit einer TLC-Wundheilungsmatrix beschichtet. Die mikroadhäsive Wundauflage trägt zu einer kontinuierlichen Wundreinigung bei und eignet sich auch für die Anwendung bei Wunden mit dem Risiko oder Anzeichen einer lokalen Infektion.* Die Wundtherapie mit UrgoClean ist auch langfristig erstattungsfähig. Dadurch leistet sie einen Beitrag für eine verlässliche Versorgung von Wundpatient:innen.
Ständige Infektionsgefahr 1
Reduziert die Bakterienlast
UrgoClean schließt Bakterien ein und hilft Bakterien aus der Wunde zu entfernen.3 Zudem absorbiert UrgoClean Exsudat, begrenzt das Mazerationsrisiko und schützt die Wundumgebung. 4, 5
Wundbeläge können jederzeit entstehen 2
UrgoClean hält die Wunde sauber. Durch den rein physikalischen Wirkmechanismus bindet und entfernt UrgoClean Fibrin.4
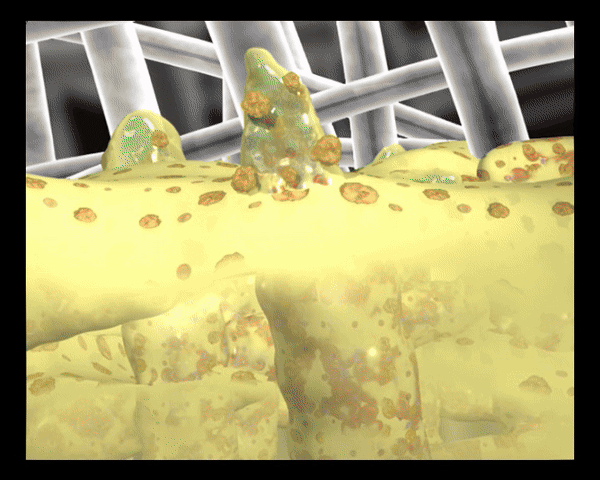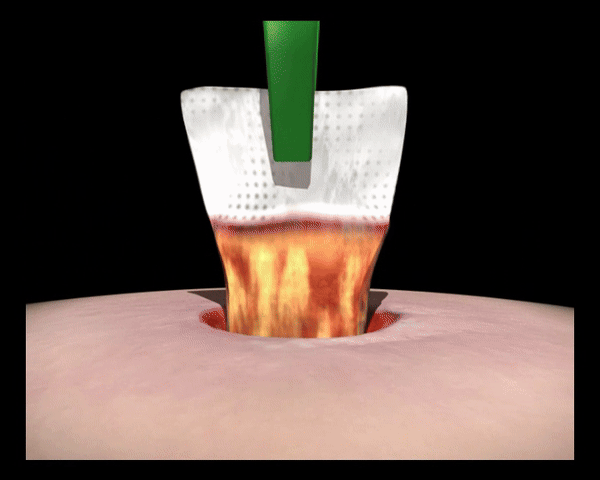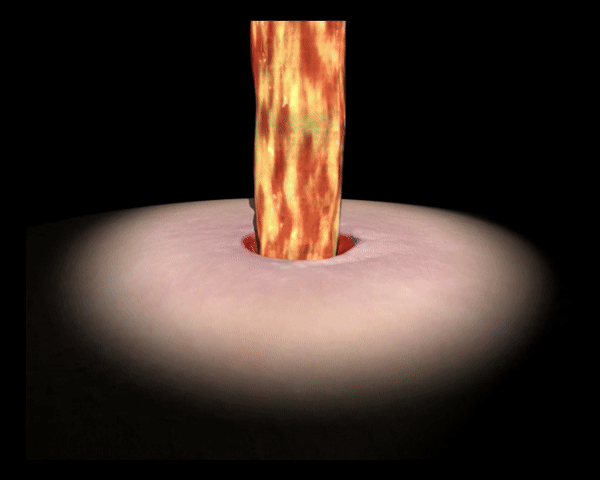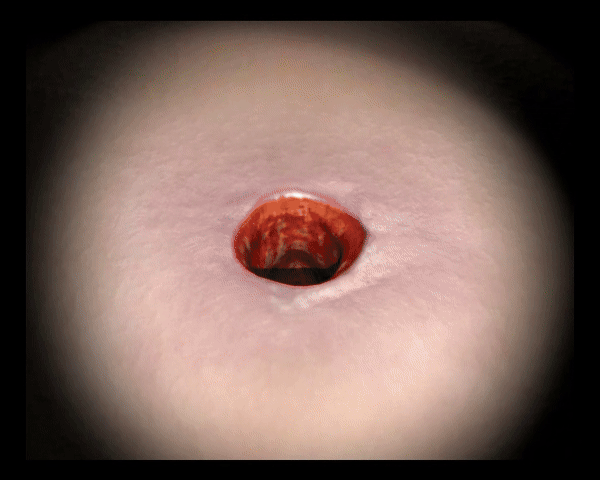
Die polyabsorbierenden Reinigungsfasern der UrgoClean Kompresse sind mit einer mikroadhäsiven TLC-Wundheilungsmatrix beschichtet, die zusammen mit den Reinigungsfasern leicht ein Gel bildet und auf diese Weise die Ableitung (Drainage) von fibrinösen Belägen sowie die Positionierung und das Ablösen der Wundauflage erleichtert.
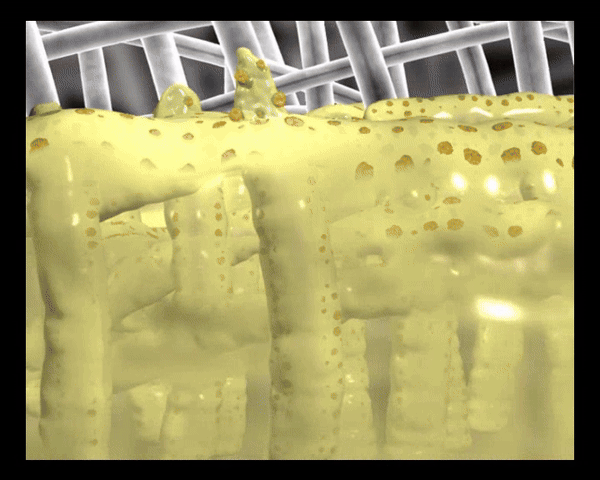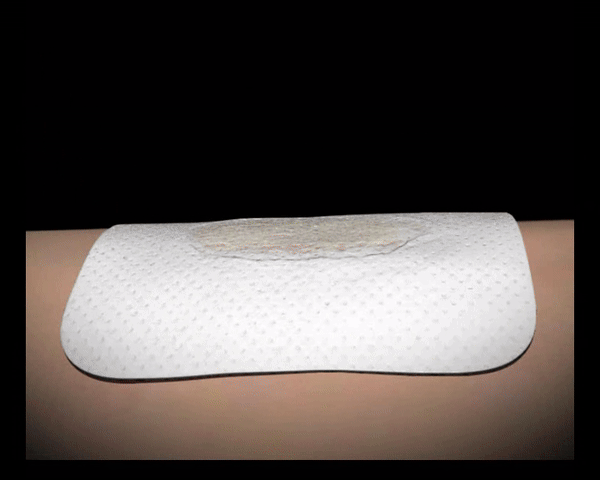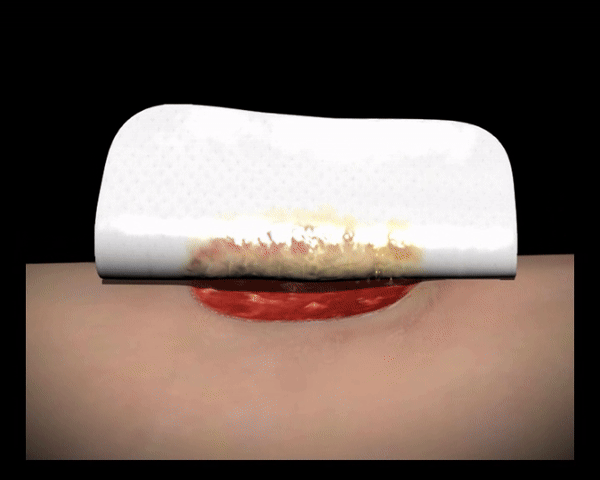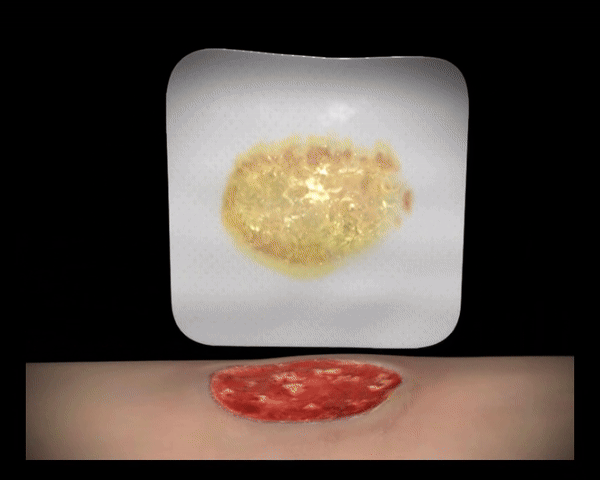
In Kontakt mit dem Wundexsudat bilden die polyabsorbierenden Reinigungsfasern ein Gel, binden fibrinöse Beläge, absorbieren diese und leiten sie in die Faserstruktur ab (autolytische Wundreinigung).

- Hilft bei der Entfernung von Bakterien aus der Wunde4
- Absorption und Retention von Exsudat und Wundbelägen5
- Hilft schwache Blutungen zu stoppen6
- Formstabil und Ablösen der Wundauflage in einem Stück7
- Schmerzfreie, atraumatische Verbandwechsel5
- Auch bei infizierten Wunden geeignet*


- Hilft bei der Entfernung von Bakterien aus der Wunde4
- Absorption und Retention von Exsudat und Wundbelägen5
- Hilft schwache Blutungen zu stoppen6
- Formstabil und Entfernung der Tamponade in einem Stück8
- Schmerzfreie, atraumatische Verbandwechsel5
- Applikationshilfe mit Tiefenmesser ermöglicht einfache Handhabung


Bindet und entfernt Fibrin durch den rein physikalischen Wirkmechanismus.

Absorbiert Exsudat, begrenzt das Mazerationsrisiko und schützt die Wundumgebung

Schließt Bakterien ein und hilft Bakterien aus der Wunde zu entfernen.

Hilft schwache Blutungen zu stoppen.

Die innovativen Produktlösungen UrgoClean Kompresse und UrgoClean Tamponade sind langfristig erstattungsfähig und tragen somit zu einer verlässlichen Versorgung von Patient:innen mit akuten oder chronischen Wunden bei.
Beispiel 1

Patientenprofil:
82-jährige Patientin mit großflächigem, stark fibrinbelegtem Ulcus cruris venosum
Zustand
Mit Ödemen und Hämatomen ins Krankenhaus eingeliefert. Wunde lokalisiert am linken Bein.
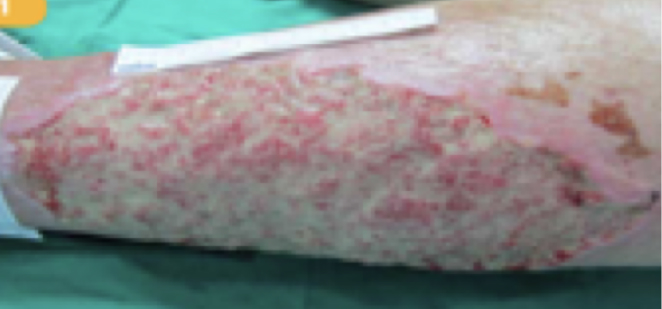
WOCHE
1
Behandlung
Behandlung mit UrgoClean Kompresse
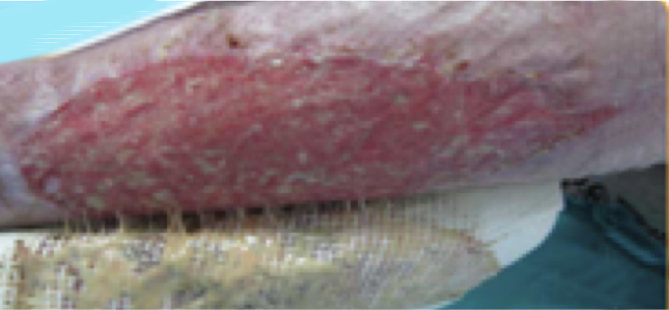
WOCHE
4
Ergebnis
Durch die lokale Wundtherapie mit UrgoClean konnte eine effektive Reinigung der Wunde erreicht werden. Die lokale Wundtherapie und Verbandwechsel waren schmerzfrei für die Patientin.
Beispiel 2

Patientenprofil:
Patientin, 83 Jahre alt, 4 Wochen altes Ulcus cruris am linken Unterschenkel lokalisiert, Größe der Wunde: 4,5 cm x 3 cm
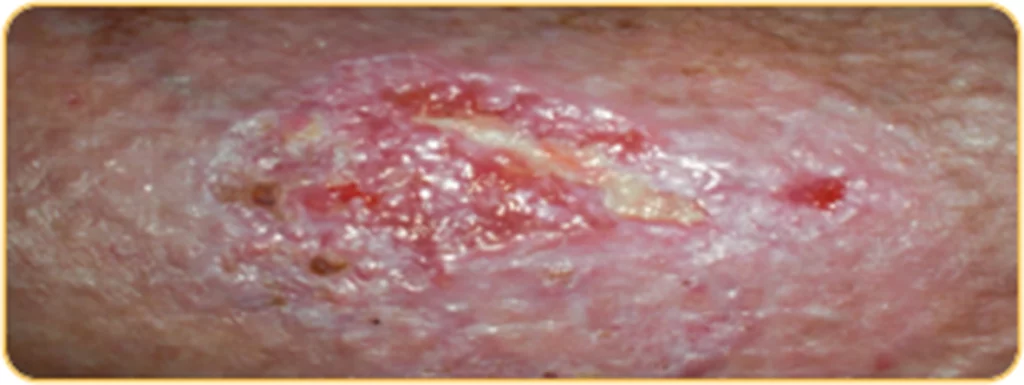
WOCHE
1
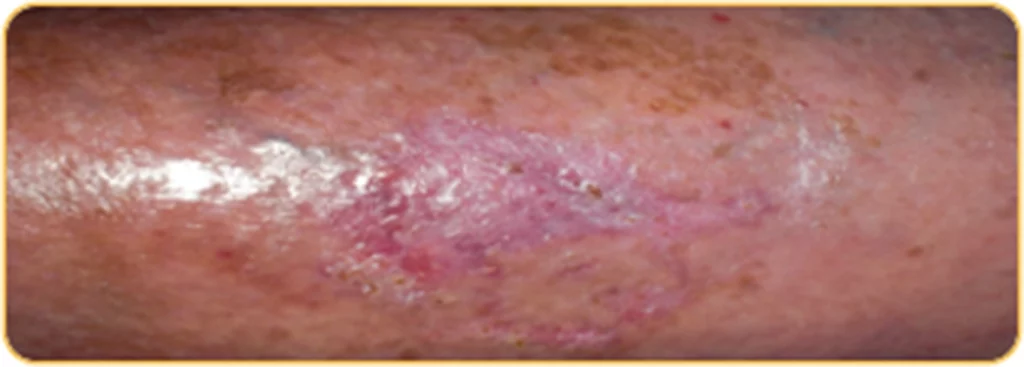
WOCHE
4


| Artikel | Abmessungen | REF | PZN |
|---|---|---|---|
| OP à 10 | 6 x 6 cm | 506448 | 08 456 260 |
| OP à 10 | 10 x 12 cm | 506449 | 08 456 277 |
| OP à 20 | 10 x 12 cm | 506441 | 08 456 283 |
| OP à 10 | 15 x 20 cm | 506450 | 08 456 308 |
| Artikel | Abmessungen | REF | PZN |
|---|---|---|---|
| OP à 5 | 5 x 40 cm/ Tamponade | 506432 | 08 456 254 |
| OP à 5 | 2,5 x 40 cm / Tamponade | 550203 | 09 763 320 |
Jetzt kostenlos Muster anfordern
FAQs
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Referenzen
1: International Wound Infection Institute (IWII) Wound Infection in Clinical Practice. Wounds International. 2022.
2: Schultz, G. et al (2017), Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Rep and Reg, 25: 744-757
3: Interne Daten, Laboratoires URGO, Rapport Nexidia 2023-01Ev2
4: Meaume S., Dissemond J,. et al. Evaluation of two fibrous wound dressings for the management of leg ulcers: Results of a European randomised controlled trial (Earth RCT). J Wound Care, Vol 23. No 3; March 2014, 105-116.
5: Meaume S., Perez J., Rethore V. et al. Management of chronic wounds with an innovative absorbent wound dressing. J Wound Care, Vol 21, no 7, July 2012, 315-322.
6: Interne Daten, Laboratoires URGO, Data on file n°88050-2009
7: Interne Daten, Laboratoires URGO, Data on file n°RE/DA/2011-185/LP, n°RS/UR/2014-017/JUB-LAP
8: Interne Daten, Laboratoires URGO, Data on file n°RS/UR/2014-017/LAP
*Aufgrund ihrer nicht okklusiven Eigenschaften kann UrgoClean unter strenger medizinischer Überwachung auch bei infizierten Wunden angewendet werden.

Diese Website verwendet Cookies (Dateien, die auf Ihrem Gerät installiert werden, wenn Sie diese Website besuchen), um die Nutzung dieser Website zu messen.
Sie können die Installation dieser Cookies zulassen oder blockieren.
Weitere Informationen zu Cookies
Die einzigen Cookies, die wir verwenden, sind Cookies zur Messung des Internetdatenverkehrs. Sie ermöglichen es uns, die Website an die Wünsche der Besucher anzupassen, dank der Messung der Anzahl der Besuche, der Anzahl der gesehenen Seiten sowie der Aktivität der Besucher auf der Website und ihrer Rückkehrrate. Wenn Sie der Hinterlegung von Cookies durch unsere Website zugestimmt haben, können Sie Ihre Zustimmung zurückziehen, indem Sie unten klicken.
| Cookie | Dauer | Beschreibung |
|---|---|---|
| cookielawinfo-checkbox-analytics | 1 year | Das Cookie wird durch die DSGVO-Cookie-Zustimmung gesetzt, um die Zustimmung des Nutzers für die Cookies in der Kategorie „Statistiken“ zu erfassen. |
| cookielawinfo-checkbox-necessary | 1 year | Das Cookie wird durch die DSGVO-Cookie-Zustimmung gesetzt, um die Zustimmung des Nutzers für die Cookies in der Kategorie „Essenziell“ zu erfassen. |
| cookielawinfo-checkbox-social-media | 1 year | Das Cookie wird durch die DSGVO-Cookie-Zustimmung gesetzt, um die Zustimmung des Nutzers für die Cookies in der Kategorie „Externe Medien“ zu erfassen. |
| Cookie | Dauer | Beschreibung |
|---|---|---|
| _ga | 2 years | Enthält eine zufallsgenerierte User-ID. Anhand dieser ID kann Google Analytics wiederkehrende User auf dieser Website wiedererkennen und die Daten von früheren Besuchen zusammenführen. |
| _gat_gtag_xxx | 1 minute | Bestimmte Daten werden nur maximal einmal pro Minute an Google Analytics gesendet. Das Cookie hat eine Lebensdauer von einer Minute. Solange es gesetzt ist, werden bestimmte Datenübertragungen unterbunden. |
| _gid | 1 day | Enthält eine zufallsgenerierte User-ID. Anhand dieser ID kann Google Analytics wiederkehrende User auf dieser Website wiedererkennen und die Daten von früheren Besuchen zusammenführen. |
